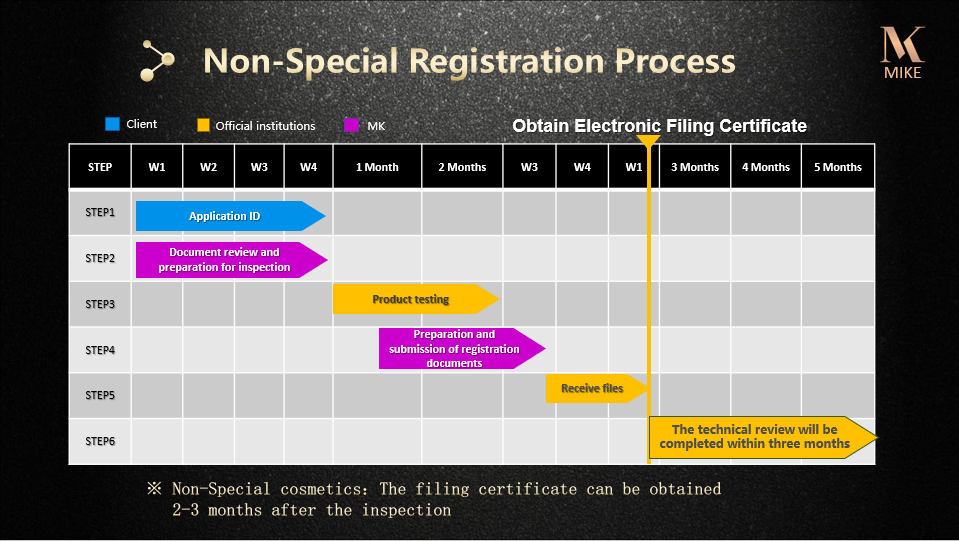
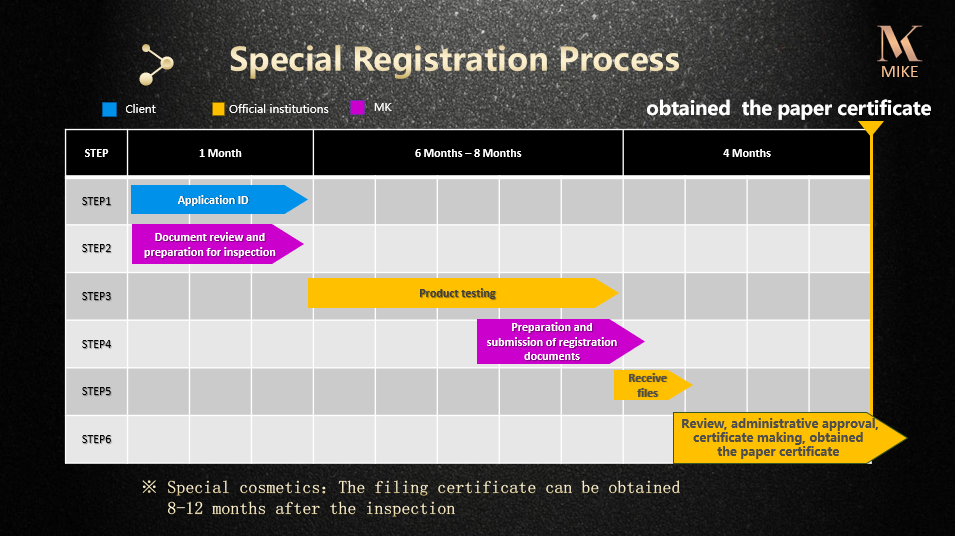
The National Medical Products Administration of the People’s Republic of China has implemented declaration and registering system for domestic special purpose cosmetic products and imported cosmetic products(special and non-special purpose). Domestic / Imported special purpose cosmetic products are required to obtain “State Food and Drug Administration – Domestic / Imported Special Purpose Cosmetic Product Health Certificate”, Imported non-special purpose cosmetic products are required to obtain “Electronic certificate for filing of imported non special purpose cosmetics”. Imported / domestic special purpose products without the registration or the filing Certificate is forbidden to sell in the mainland of China. Since November 10, 2018,the Filing Certificate of imported non-special cosmetics shall be changed into electronic certificate.
Categor | Introduction | Management System |
|---|---|---|
| (Non-Special) | 发用/洁面产品 (Hair/FaceCleansing) | Filing |
| 一般护肤用品 (Common Skin Care) | ||
| 易触及眼睛护肤用品 (Eye Contacting Skin Care) | ||
| 一般彩妆品 (Common Make-up) | ||
| 眼部彩妆品 (Eye Make-up) | ||
| 护唇及唇部彩妆品 (Lip Make-up) | ||
| 指(趾)甲用品 (Nail) | ||
| 芳香品 (Fragrance) | ||
| 特殊类 | 育发类 (Hair Growth) | Registration |
| (Special) | 健美类 (Slimming) | |
| 美乳类 (Breast Beautifier) | ||
| 染发类 (Hair Dyes) | ||
| 烫发类 (Perm) | ||
| 防晒类 (Sunscreen) | ||
| 除臭类 (Deodorant) | ||
| 美白祛斑类(不触及眼部) (Whitening & Spot Removal Products)[non eye contacting] | ||
| 美白祛斑类(触及眼部)(Whitening & Spot Removal Products)[eye contacting] | ||
| 脱毛类 (Depilatories) |
-
01
Phase 1
1. The information table (we will provide this)
2. Business license of the correspondent Chinese liability entity (digital)
3. Business license of the foreign entity (digital)
4. Product formula: INCI standard formula
5. Product packaging design file (pdf or AI)
6. Product packaging translation in English or Chinese
7. Product sample (2 of each) [this is not the samples for testing]
-
02
Phase 2:
1. Manufacture process sketch and brief description
2. Certificate of free sales (original copy)
3. GMP/ISO (original copy)
4. Consigned processing agreement (not required if isn’t cosigned processing relationship)